CRISPR/Cas-based genome editing has dramatically improved genetic modification technology. In situ electroporation called genome editing via oviductal nucleic acid delivery (GONAD), which eliminates the need for ex vivo embryo handling, is technically the simplest method for gene transfer and can be performed in laboratories without developmental engineering expertise including micromanipulation techniques. However, the use of this method remains challenging in the case of large-fragment knock-in, such as gene expression cassettes. Adeno-associated viruses (AAV) act as donor DNA for homologous recombination in infected cells, including rodent embryos. Simultaneous electroporation of AAV donors and CRISPR/Cas9 components into embryos to create knock-in animals, and successfully generated knock-in rats carrying a gene cassette with a Large-fragment.
AAV is known to function as a donor DNA for homologous recombination (HR). After ex vivo AAV infection of embryos, a method for knock-in of multikilobase sequences was developed. AAV along with CRISPR/Cas9 components could be immediately and physically introduced into cells by electric pulses and function as donor DNAs.
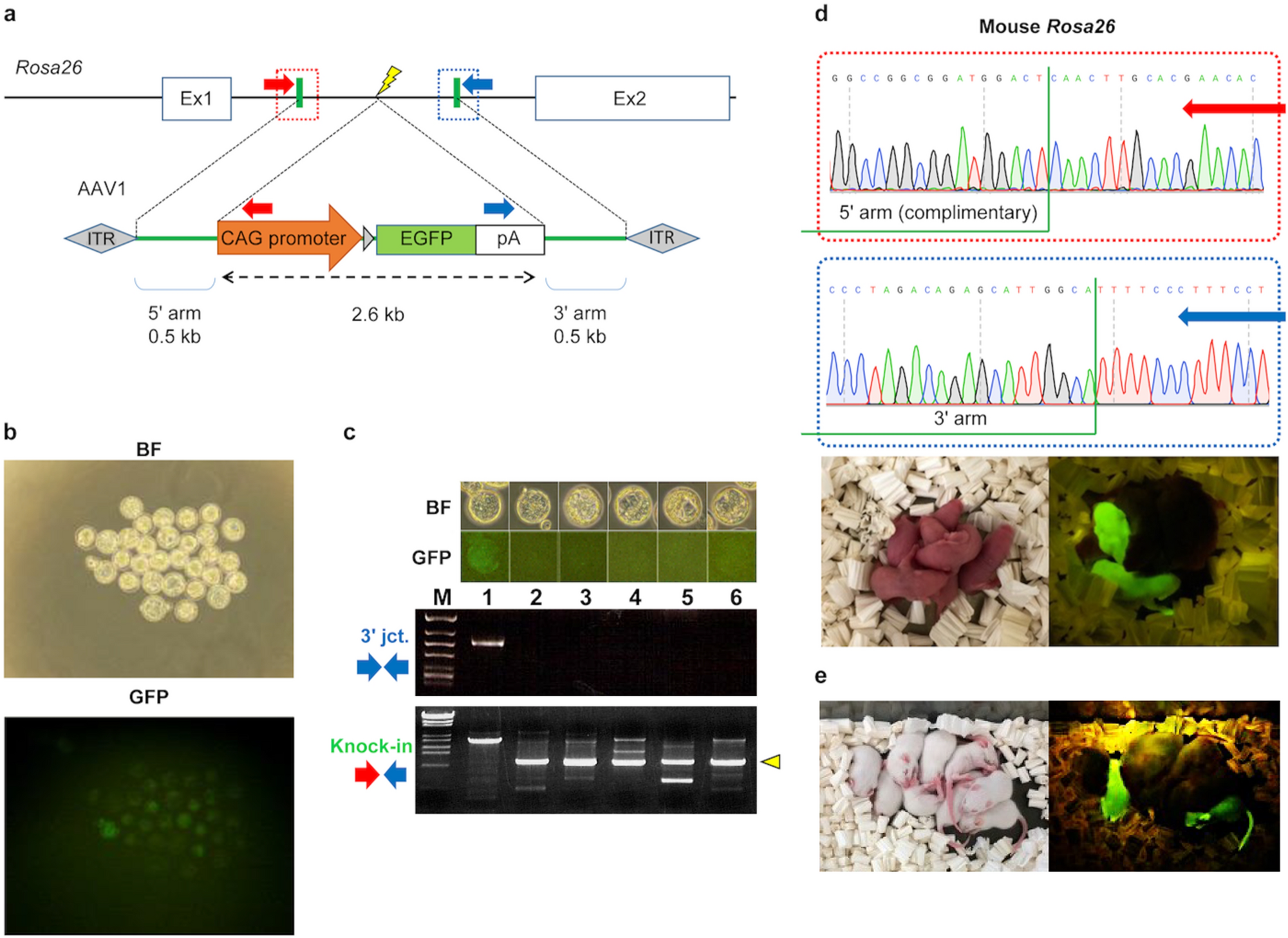
Generation of knock-in mice by simultaneous electroporation of Cas9 RNP and AAV vectors ex vivo.
Using AAV vectors of serotypes 2, 5, and 6, CRISPR RNPs were also electroporated into embryos, and the knock-in efficiency was confirmed using genomic PCR.
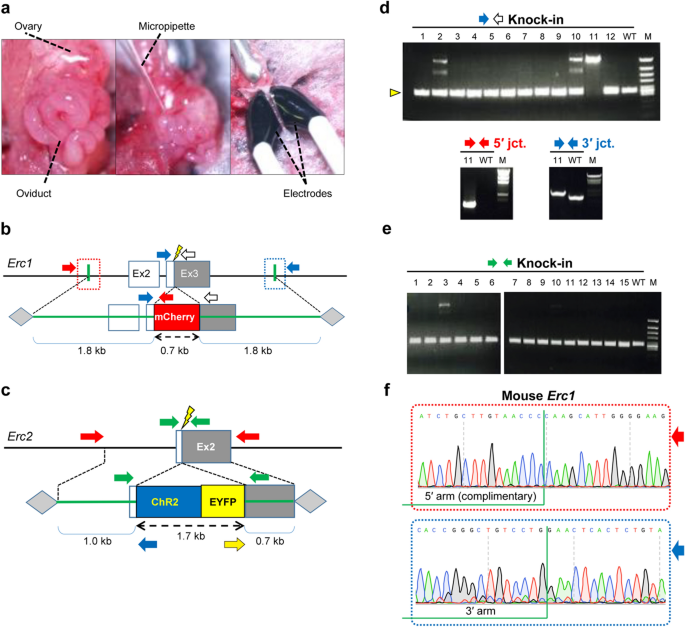
Generation of knock-in mice by in situ electroporation using AAV1 donors.
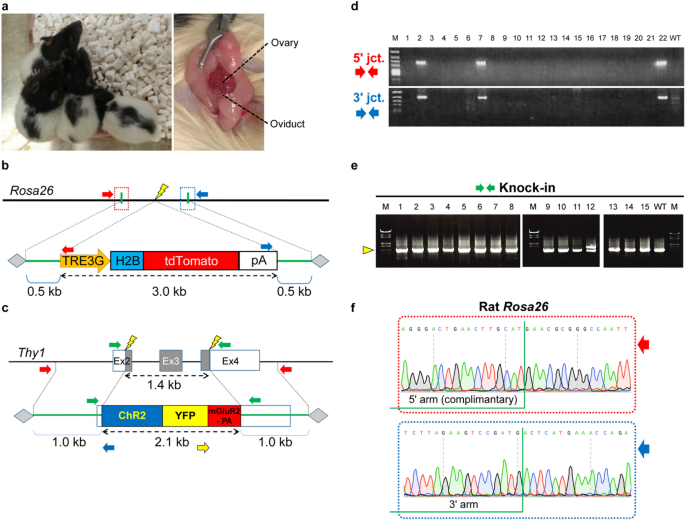
Generation of knock-in rats carrying a gene cassette by in situ electroporation of AAV1 donors.
AAV vectors along with CRISPR/Cas9 components through in situ electroporation, resulting in a knock-in of roughly 3 kb. This strategy will offer fantastic opportunities to clarify the molecular mechanisms of higher-order life phenomena.
The current study used in situ electroporation of AAV donors and CRISPR/Cas9 components into embryos to create knock-in animals with large-fragment insertions. Both large-scale projects and small-scale lab experiments can produce functional knock-in animals that contain gene expression cassettes with the help of this methodology. Investigating experimental variables like the concentration of AAV donors or the make-up of CRISPR solutions could lead to further improvements in knock-in efficiency.
1. Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
2. Honda, A. et al. Single-step generation of rabbits carrying a targeted allele of the tyrosinase gene using CRISPR/Cas9. Exp. Anim. 64, 31–37 (2015).
3. Gu, B., Posfai, E., Gertsenstein, M. & Rossant, J. Efficient generation of large-fragment knock-in mouse models using 2-cell (2C)-homologous recombination (HR)-CRISPR. Curr. Protoc. Mouse Biol. 10, e67 (2020).
4. Namba, M. et al. GONAD: A new method for germline genome editing in mice and rats. Dev. Growth Differ. 63, 439–447 (2021).
5. Yoshimi, K. et al. Combi-CRISPR: Combination of NHEJ and HDR provides efficient and precise plasmid-based knock-ins in mice and rats. Hum. Genet. 140, 277–287 (2021).


